Background: The combination of BCL2 and BTK inhibitors has shown synergistic activity. The combination of venetoclax, a BCL2 inhibitor, and ibrutinib, a BTK inhibitor, is an effective treatment for CLL/SLL. Sonrotoclax (BGB-11417) is a BH3 mimetic that binds and inhibits BCL2 with potency >10x that of venetoclax in biochemical assays. Zanubrutinib, a next-generation BTK inhibitor, has shown improved PFS with fewer cardiac adverse events (AEs) vs ibrutinib in a randomized study of patients with CLL/SLL (Brown et al. N Engl J Med. 2023). BGB-11417-101 (NCT04277637) is an ongoing, first-in-human, phase 1/1b dose-escalation/expansion study of patients with various B-cell malignancies. In a preliminary report, sonrotoclax, alone or in combination with zanubrutinib, was well tolerated at all doses tested up to 640 mg. This abstract describes the safety and efficacy data from patients with treatment-naïve (TN) CLL/SLL who received the combination of sonrotoclax and zanubrutinib.
Methods:Patients received zanubrutinib (320 mg QD or 160 mg BID) 8-12 weeks before starting sonrotoclax using a ramp-up schedule starting from 1 mg to the intended target dosage of 160 mg or 320 mg QD (doubling weekly [W] or 30% increase every day 5 d/week [D]) to mitigate risk of tumor lysis syndrome (TLS). Patients were treated until progression, unacceptable toxicity, or could elect to stop. TLS was assessed per Howard (2011) criteria; mitigation included mandatory oral hydration and antihyperuricemics. Primary endpoint was safety (reported per CTCAEs v5.0); a secondary endpoint was ORR (per iwCLL 2008 criteria) and minimal residual disease assessed in blood by ERIC flow every 24 weeks (uMRD4; <1 CLL cell per 10,000 leukocytes, or <0.01%) was an exploratory endpoint.
Results: As of May 21, 2023, 94 patients with TN-CLL/SLL were enrolled (sonrotoclax 160 mg QD, n=41 [26 W, 15 D] and 320 mg QD, n=53 [25 W, 28 D]); 15 patients were still receiving zanubrutinib monotherapy and 79 started sonrotoclax (160 mg, n=32 [25 W, 7 D] and 320 mg, n=47 [24 W, 23 D]). Median follow-up was 8.5 mo (range: 0.6-18.2) for all patients; 160 mg, 12.1 mo (range: 0.6-18.2); 320 mg, 7.0 mo (range: 1.1-14.6). No deaths have occurred, and all patients remain on study.
Treatment-emergent AEs (TEAEs) occurring in ≥20% patients who received sonrotoclax plus zanubrutinib (n=79) are listed in theTable; TEAE frequencies were similar between the two sonrotoclax cohorts. Contusion, neutropenia, and low-grade gastrointestinal toxicity were the most common TEAEs; neutropenia was the most common grade ≥3 TEAE (n=13 [17%]). No cases of clinical or laboratory TLS occurred in either ramp-up schedule; no patient experienced atrial fibrillation. One TEAE (cryptococcal meningitis at 11 weeks treated with azoles) led to treatment discontinuation. Sonrotoclax dose holds occurred in 17 patients (22%) for a median duration of 11 days (range: 3-37) and three patients (4%) had dose reduction. The most common TEAEs resulting in dose holds were COVID-19 (n=9 [11%]) and diarrhea (n=3 [4%]).
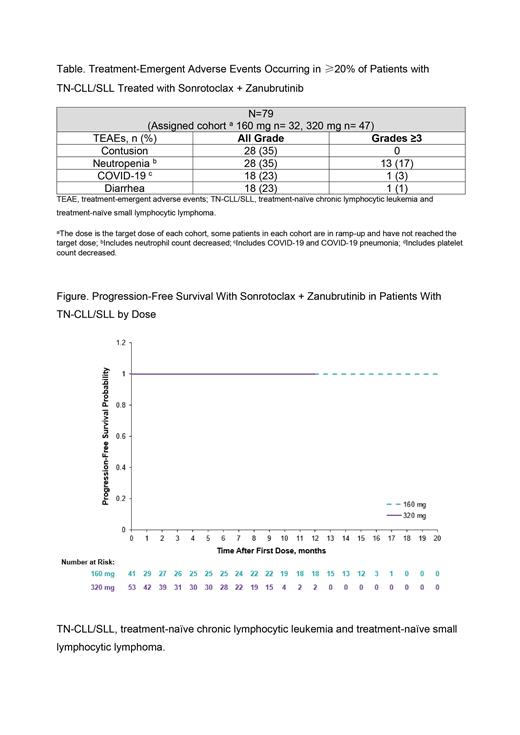
A total of 56 patients had a response assessment. The ORR was 100% (CR: 160 mg, n=9 [36%]; 320 mg, n=6 [19%]). Across all doses, rate of CR increased with time; overall median time to CR was 10.1 mo (range: 5.4, 17.1). No progression was reported in either cohort (Figure). Week 24 blood uMRD4 rates were 50% (n=12/24) in 160 mg and 65% (n=13/20) in 320 mg patients. Week 48 blood uMRD4 rates were 73% in 160 mg (n=11/15) and 100% (n=1/1) in 320 mg; no patient has lost uMRD4.
Conclusion: Sonrotoclax (160 mg and 320 mg) in combination with zanubrutinib was well tolerated in patients with TN CLL/SLL. Only one patient discontinued treatment and three patients had dosage reductions. No TLS was seen with either ramp-up schedule. Acknowledging the short follow up, efficacy is encouraging with 100% ORR in assessed patients and no PFS events. High rates of blood uMRD4 occurred early. Based on these data, a phase 3 study assessing this combination is planned.
Disclosures
Tam:Roche: Honoraria; Novartis: Honoraria; LOXO: Honoraria; Beigene: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding. Anderson:National Health and Medical Research Council: Research Funding; Roche: Honoraria; Novartis: Honoraria; Takeda: Honoraria; Kite: Honoraria; AstraZeneca: Honoraria; Beigene: Honoraria; Abbvie: Honoraria; Janssen: Honoraria; The Walter and Eliza Hall Institute: Patents & Royalties. Lasica:JanssenJanssen: Other: Education; Celgene: Other: TRAVEL, ACCOMMODATIONS, EXPENSES. Verner:Janssen Cilag Pty Ltd: Research Funding. Opat:Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astra Zeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Beigene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen Cilag: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck Sharpe & Dohme: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Honoraria, Research Funding; Roche: Honoraria, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees; CSL Behring: Membership on an entity's Board of Directors or advisory committees; Antengene: Membership on an entity's Board of Directors or advisory committees. Ma:Genentech: Consultancy; Janssen Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Juno/BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Consultancy, Research Funding; Eli Lilly and Company/Loxo Oncology: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Weinkove:AbbVie: Honoraria; BeiGene: Honoraria; BioOra: Research Funding; Fisher & Paykel Healthcare: Current equity holder in publicly-traded company; Janssen: Honoraria, Research Funding. Cordoba:European Hematology Association (EHA), Spanish Society Hematology (SEHH): Membership on an entity's Board of Directors or advisory committees; F. Hoffmann-La Roche Ltd, Takeda, Abbvie, Janssen, AstraZeneca, Lilly, BeiGene, BMS, Genmab, Incyte, Gilead: Speakers Bureau; F. Hoffmann-La Roche Ltd, Takeda, Abbvie, Janssen, AstraZeneca, Lilly, BeiGene, BMS, Genmab, Incyte, Gilead: Consultancy; Fundacion Jimenez Diaz University Hospital: Current Employment. Soumerai:Adaptive Biotechnologies, Beigene, BostonGene, Genentech/Roche, GlaxoSmithKline, Moderna, Takeda, TG Therapeutics: Research Funding; AstraZeneca, Beigene, Biogen, Bristol Myers Squibb, Roche, Seattle Genetics: Consultancy. Ghia:Lilly/Loxo Oncology: Consultancy, Honoraria, Research Funding; MSD: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding; BeiGene: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding. Hilger:BeiGene: Current Employment; BeiGene: Current equity holder in publicly-traded company. Fang:BeiGene: Current Employment; BeiGene: Current equity holder in publicly-traded company. Simpson:BeiGene: Current Employment; BeiGene: Current equity holder in publicly-traded company. Guo:BeiGene: Current equity holder in publicly-traded company; BeiGene: Current Employment. Cheah:MSD: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; TG therapeutics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; AstraZenecca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Lilly: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Ascentage Pharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BeiGene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Research Funding; Menarini: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: TRAVEL, ACCOMMODATIONS, EXPENSES, Research Funding; Genmab: Consultancy, Honoraria; Daizai: Consultancy, Honoraria.